
Cultivation & Production
The cultivation of special crops requires comprehensive know-how, specific infrastructure and, due to the often long-term nature of the crops, having land or reliable cultivation partners.
The constantly increasing demands for higher quality and comprehensive documentation for raw materials and the often-limited availability of raw materials necessitate trusting and transparent cooperation. Open and full communication forms the basis for successful cooperation.
Our services
After completion of the pilot phase, large-scale cultivation begins either on one of our farms or at suitable partner company. This closely controlled cultivation can take place anywhere around the globe, depending on the cultivation conditions required.
All production processes from the establishment of a field to the finished packaged raw material are precisely documented. In this way we ensure the control and traceability of the product. The cultivation of the crops takes place strictly in accordance with the WHO guidelines of the "Good Agricultural and Collection Practice" (GACP).
The constantly increasing official requirements concerning the purity of the raw material represent a major challenge in the industry. Vitaplant focuses on the quality of the raw material in production. This includes in particular the prevention of impurities such as the introduction of pyrrolizidine alkaloid-containing weeds, the prevention of PAHs (polycyclic aromatic hydrocarbons) or exposure to pesticides.
In close cooperation with our customers, we seek to develop ways to guarantee a specific supply as well as a permanent improvement of the raw material.
Cultivation – Subsidiaries
The cultivation of special crops represents a demanding undertaking during all the production steps – from the propagation of young plants to post-harvest treatment. The cultivation requires extensive knowledge and experience, entrepreneurship and often a fair amount of risk.
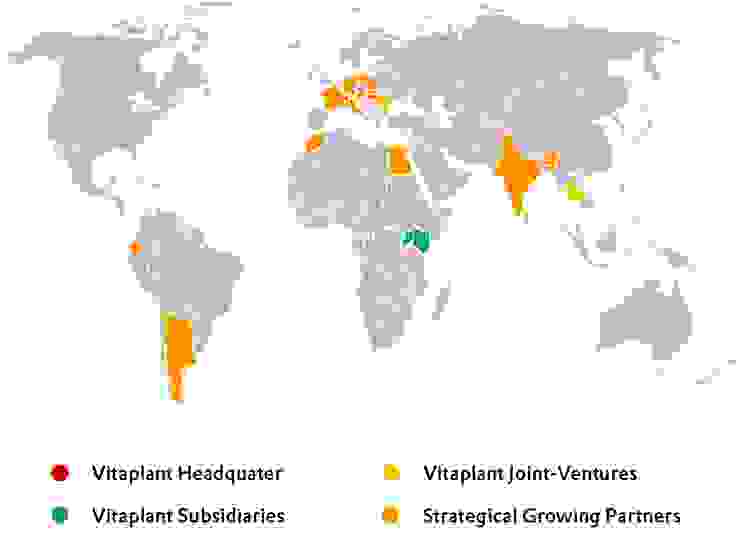
Vitaplant has established subsidiaries in various geographical regions to cover these needs. Local management and long-term leased cultivated land as well as extensive investments in infrastructure guarantee flexibility, responsiveness to specific customer needs as well as the production of high-quality raw materials.
Direct influence and close controls by Vitaplant experts as well as a constant exchange with the local management team ensure the highest quality and transparency at all stages of the cultivation process.
Network of international production partners
In addition to its subsidiaries, Vitaplant has at its disposal a broad network of international cultivation partners with the ability to grow crops on a large scale in a wide variety of climatic conditions.
Vitaplant portfolio
The following table provides an excerpt from the Vitaplant portfolio. Countless other cultures have been researched and cultivated over the past 25 years.
Do you have special requirements? Contact us!
| Scientific name | Name | Used part |
|---|---|---|
| Achillea millefolium | Yarrow | herba |
| Arctostaphylos uva-ursi | Bearberry leaves | herba |
| Alchemilla vulgaris | Lady's mantle | herba |
| Artemisia absinthium | Wormwood | herba |
| Avena sativa | Oats | herba |
| Calendula officinalis | Marigold | flos / herba |
| Cannabis sativa | Cannabis | flos / herba |
| Centaurea cyanus | Cornflower | flos |
| Commiphora myrrha | Myrrh | resina |
| Echinacea pur. / angu. / pal. | Echinaceas | herba / radix |
| Hyoscyamus niger | Henbane | herba |
| Hypericum perforatum | St. John's wort | herba |
| Malva sylvestris | Mallow | flos / herba |
| Matricaria chamomilla | Chamomile | flos |
| Melissa officinale | Lemon balm | herba |
| Moringa oleifera | Moringa | herba |
| Orthosiphon aristatus | Javatee | herba / folia |
| Passiflora incarnata | Passionflower | herba |
| Plantago lanceolata | Plantain | herba |
| Salvia officinalis | Sage | herba |
| Solidago virgaurea | Goldenrot | herba |
| Taraxacum officinale | Dandelion | herba / radix |
| Thymus vulgaris | Thyme | herba / folia |
| Urtica dioica | Nettel | herba |
| Valeriana officinalis | Valerian | radix |
| Vitex agnus-castus | Chaste Tree | fructus |
Consulting and sales
We would be happy to discuss the requirements for your plant raw materials.
Talk to our experts.
Contact details






